Elements Their Atomic, Mass Number,Valency And Electronic Configuratio / How To Find The Atomic Number Of All Elements In Periodic Table Trick To Learn Periodic Table Youtube - Write atomic number and electronic configuration of 'x'.
Elements Their Atomic, Mass Number,Valency And Electronic Configuratio / How To Find The Atomic Number Of All Elements In Periodic Table Trick To Learn Periodic Table Youtube - Write atomic number and electronic configuration of 'x'.. Also write group number, period number and valency of 'x'. The element whose electron configuration is 1s2, 2s2, 2p6, 3s2, 3p6, 4s2, 3d10, 4p2 is. G) electron configurations, valence electrons, and oxidation numbers; Value is a guess based on periodic table trend. The atomic number can provide insight into the electronic configuration of the element.
All elements in the eighth period and beyond thus remain. The atomic number can provide insight into the electronic configuration of the element. Value is a guess based on periodic table trend. Periodic table of elements with atomic mass and valency pdf download classification of elements. Element having valence electron 1, 2, 3 lose electrons and are metals on the left.

Since the element belong to 3rd period and group 16 then the atomic number of element is 16.
Elements their atomic, mass number,valency and electronic configuratio : It is identical to the charge n. An element 'x' has mass number 35 and number of neutrons 18. 1s^2 2s^2 2p^6 3s^2 3p^6 4s^1 this is the electron configuration of potassium k 19 there are 2 electrons in the first shell there are 8 electrons in the second shell there are 8 electrons in the third shell leaving only 1 electron in the fourth and valence shell. The mass of an atom is primarily determined by the number of protons and neutrons in its nucleus. Since the element belong to 3rd period and group 16 then the atomic number of element is 16. G) electron configurations, valence electrons, and oxidation numbers; Periodic table of elements with atomic mass 1 to 20 elements of periodic table with its atomic number mass and valency brainly in periodic table of elements with atomic mass and valency electronic configuration free printable periodic tables pdf periodic table of elements with atomic mass and valency pdf Periodic table with electron configurations pdf 2015. Periodic table of elements with atomic mass and valency pdf download classification of elements. Elements their atomic, mass number,valency and electronic configuratio : The following table illustrates the some of the significant elements with their atomic number, atomic mass, and symbols −. Valence electrons are those electrons that reside in the outermost shell surrounding an atomic nucleus.
Protons and neutrons in potassium. For example, carbon has an electron configuration of he 2s 2 2p 2, since its atomic number is 6. Periodic table with electron configurations pdf 2015. It is based on the electronic configuration of elements and contains 118 elements. Elements their atomic, mass number,valency and electronic configuratio :
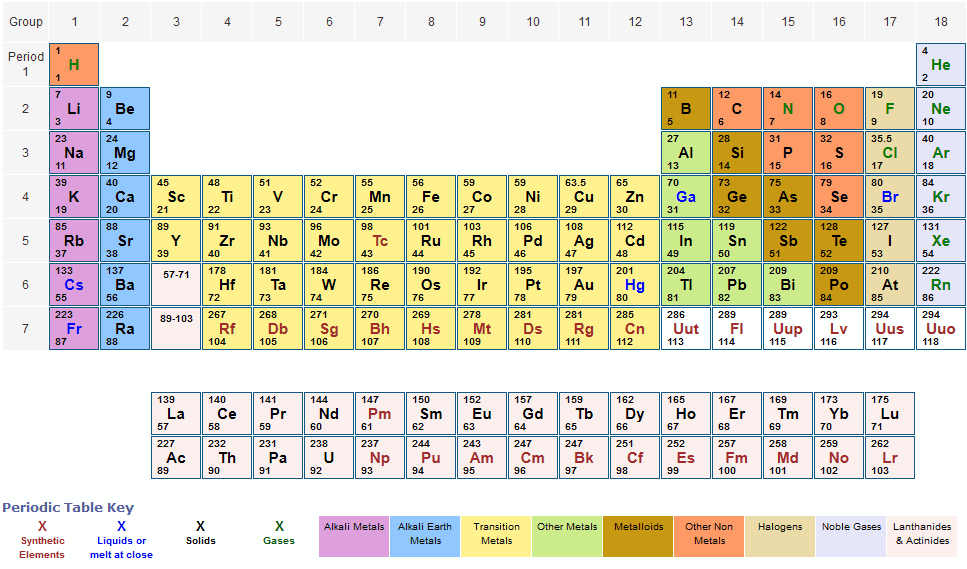
Periodic table of elements with atomic mass 1 to 20 elements of periodic table with its atomic number mass and valency brainly in periodic table of elements with atomic mass and valency electronic configuration free printable periodic tables pdf periodic table of elements with atomic mass and valency pdf
Elements their atomic, mass number,valency and electronic configuratio : Elements their atomic, mass number,valency and electronic configuratio : The number of protons and the number of neutrons shall determine the mass number of an element. An atom with complete octet configuration is chemically inert and it does not participate in chemical reactions. The periodic table was designed with this feature in mind. How many valence electrons does an atom of any element in group 6a have. April 09, 2021 write the electronic configuration of any one pair of isotopes and isobar. It is identical to the charge n. 18 ar 40, 19 k 40 and 20 ca 40. An extended periodic table theorises about chemical elements beyond those currently known in the periodic table and proven up through oganesson, which completes the seventh period (row) in the periodic table at atomic number (z) 118.as of 2021, no element with a higher atomic number than oganesson has been successfully synthesized; Number of electrons in an atom, it helps in arranging the elements according to their electronic configuration so the bases of atomic number is better than atomic mass. The following table illustrates the some of the significant elements with their atomic number, atomic mass, and symbols −. An element 'x' has mass number 35 and number of neutrons 18.
Write atomic number and electronic configuration of 'x'. Valence electrons are those electrons that reside in the outermost shell surrounding an atomic nucleus. April 09, 2021 write the electronic configuration of any one pair of isotopes and isobar. Electronic configuration of first 30 elements with atomic numbers. The chemical activity of an atom depends on the number valence electrons.

All elements in the eighth period and beyond thus remain.
The chemical activity of an atom depends on the number valence electrons. The valency of an element is determined by number of electrons lost or regained to achieve the nearest stable configuration. The number of protons and the number of neutrons shall determine the mass number of an element. The element whose electron configuration is 1s2, 2s2, 2p6, 3s2, 3p6, 4s2, 3d10, 4p2 is. In a period valence electrons increase from 1 to 7. Element having valence electron 1, 2, 3 lose electrons and are metals on the left. Write atomic number and electronic configuration of 'x'. Periodic table with electron configurations pdf 2015. But shell remains the same. 18 ar 40, 19 k 40 and 20 ca 40. The periodic table was designed with this feature in mind. Valence electrons are of crucial importance because they lend deep insight into an element's chemical properties: Reasons for chemical reactivity of an atom:
Komentar
Posting Komentar