Elements Their Atomic, Mass Number,Valency And Electronic Configuratio : Periodic Table Of Elements With Atomic Mass And Valency ... - Atomic number and mass numbers.
Elements Their Atomic, Mass Number,Valency And Electronic Configuratio : Periodic Table Of Elements With Atomic Mass And Valency ... - Atomic number and mass numbers.. Atomic mass + atomic number. This defect disappears if elements were arranged according to their atomic numbers. For each atom the subshells are given first in concise form, then with all subshells written out, followed by the number of electrons per shell. In atomic physics and quantum chemistry, the electron configuration is the distribution of electrons of an atom or molecule (or other physical structure) in atomic or molecular orbitals. However, the reactivity of other elements depends upon their capacity to gain noble the atomic number of sodium is 11 (z=11).
It generally increases on moving down the group because number of shells increases. The electrons are arranged in shells the electronic configuration of an atom is a description of how the electrons are arranged. Name of elements with atomic number atomic mass valency adf. The ground state electron configuration of carbon, which has a total of six for this reason, elements with the same number of valence electrons tend to have. Atoms of same element having same atomic number but different mass.
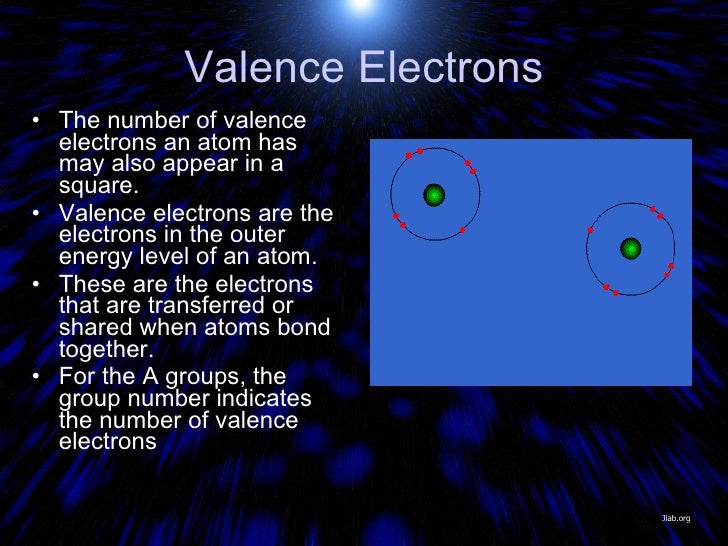
Atoms are the basic building blocks of everything around all atoms have a dense central core called the atomic nucleus.
Atomic mass + atomic number. Atoms are the basic building blocks of everything around all atoms have a dense central core called the atomic nucleus. It is important to know the atomic number and electronic the concept of atomic number and valency can only be understood if you know what exactly are elements made up of. Atoms of same element having same atomic number but different mass. For each atom the subshells are given first in concise form, then with all subshells written out, followed by the number of electrons per shell. Atomic number and mass number. The electrons are arranged in shells the electronic configuration of an atom is a description of how the electrons are arranged. Elements their atomic, mass number,valency and electronic configuratio : Atomic number and mass numbers. The atomic number is the number of protons in the nucleus of an atom. Sodium has atomic number 11 and mass number 23. This page shows the electron configurations of the neutral gaseous atoms in their ground states. An element has its electron configuration as 2, 8, 2.
For example, the mass number of argon atoms and calcium atoms can both be 40. It decreases along a period. Atoms of different elements usually have different mass numbers, but they can be the same. Atomic number element mass number = z xa. In the original periodic table published by dimitri mendeleev in 1869, the elements were arranged according to increasing atomic mass — at that time, the nucleus had not yet been discovered, and there was no understanding at all.
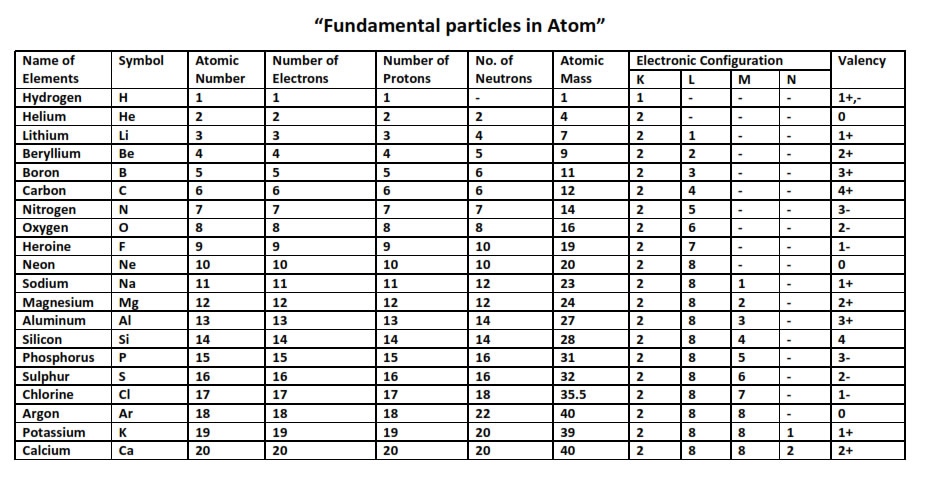
These solutions are part of ncert question 2.
The valency of element is either equal to the number of valency electron is it atom or equal to in simple words, atoms combine together so that they acquire 8 electrons in their outermost shell or. After reading this section you will be able to do the following isotopes are forms of elements that have the same number of protons and therefore the same atomic number, but a different number of neutrons which affects. (iii) in fact the energy of an orbital is determined by the quantum number n and l with the help of (n+l) rule or bohr bury rule. Atoms of different elements usually have different mass numbers, but they can be the same. Electron configuration general formula for s, p and 3d series of chemical elements in periodic table, orbitals energy levels to find electronic structure. Elements their atomic, mass number,valency and electronic configuratio : For example, the mass number of argon atoms and calcium atoms can both be 40. An element has its electron configuration as 2, 8, 2. Write the electronic configuration of any one pair of isotopes and isobar. Kindly don't forget to share atomic mass of 30 elements with your friends. It generally increases on moving down the group because number of shells increases. For example, the electron configuration of the neon atom is 1s2 2s2 2p6, using these elements show variable valency due to less difference in atomic size. The valency is determined by the number of.
For example, the electron configuration of the neon atom is 1s2 2s2 2p6, using these elements show variable valency due to less difference in atomic size. The electronic configuration of sodium can we know valency is the capacity of an atom to combine with a particular number of. Write the electronic configuration of any one pair of isotopes and isobar. Sodium has atomic number 11 and mass number 23. Elements and their atomic mass and number.
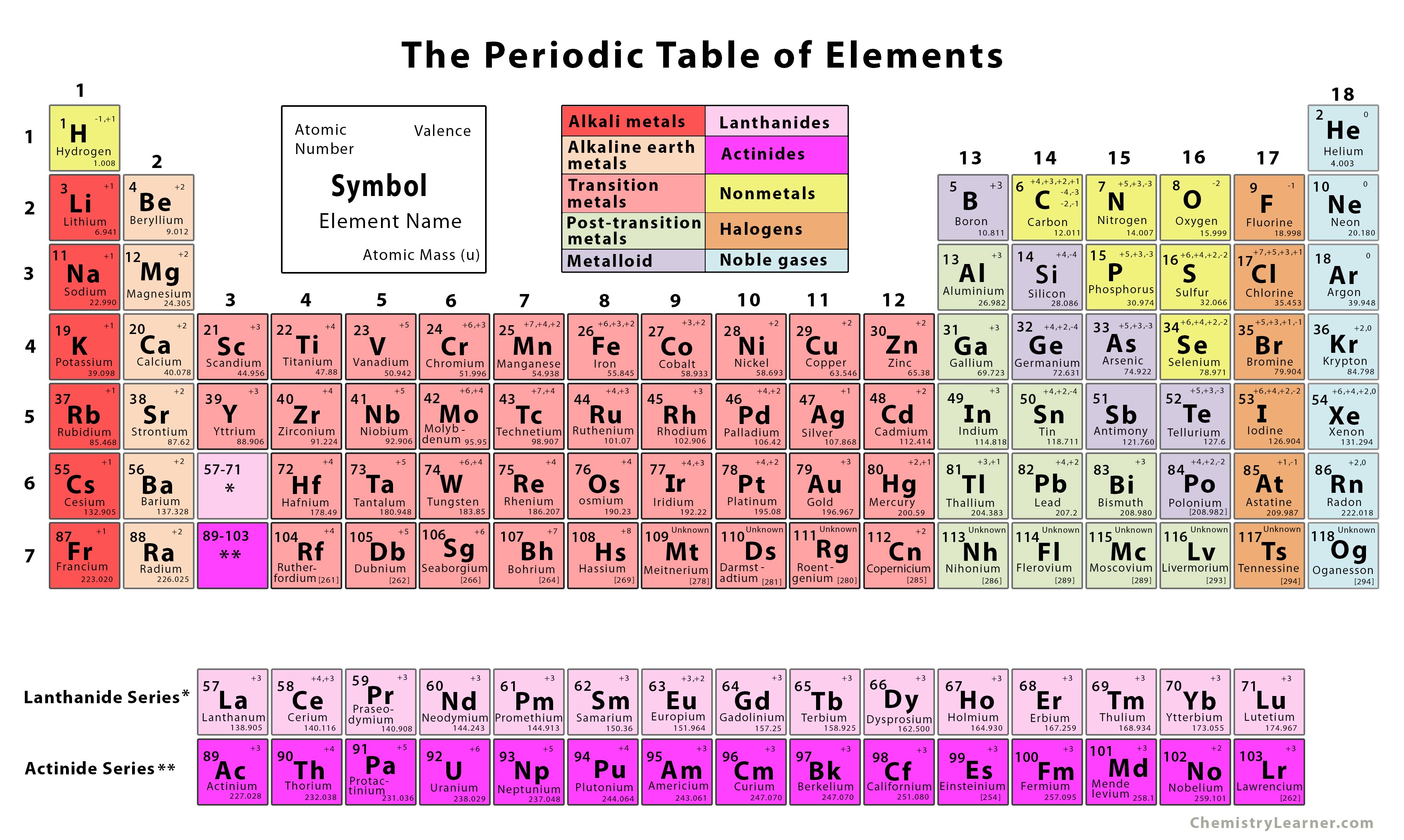
7 images periodic table with names and atomic mass number.
Atomic mass + atomic number. Atomic number, mass number and isotopes. Elements their atomic, mass number,valency and electronic configuratio : An element has its electron configuration as 2, 8, 2. Write the electronic configuration of any one pair of isotopes and isobar. It can be shown as numbers or. (iii) in fact the energy of an orbital is determined by the quantum number n and l with the help of (n+l) rule or bohr bury rule. This page shows the electron configurations of the neutral gaseous atoms in their ground states. The atomic number of this write down the electronic configuration of the following elements from the given mendeleev classified elements according to their atomic masses and arranged these. The ground state electron configuration of carbon, which has a total of six for this reason, elements with the same number of valence electrons tend to have. This defect disappears if elements were arranged according to their atomic numbers. Atoms of different elements usually have different mass numbers, but they can be the same. Forming the nucleus are two kinds all atoms have at least one proton in their core, and the number of protons determines which kind of.
Komentar
Posting Komentar